The AspireAssist, a New FDA-approved Weight Loss Solution
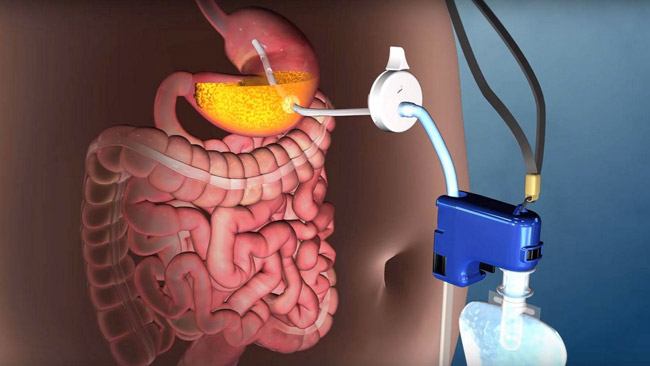
A new weightloss solution which allows the patient to remove about 30% of the food from the stomach before the calories are absorbed into the body, assisting weight loss.
A device developed by Aspire Bariatrics, helps weight loss in obese patients with a body mass index (BMI) of 35 to 55, who have been unsuccessful in losing weight with nonsurgical weight-loss therapies.
What does the Procedure Involves?
A thin tube is endoscopically placed through an incision in the skin into the stomach. The quick 15 minute procedure is performed under "twilight anesthesia" - general anesthesia is usually not required. The tube connects the inside of your stomach directly to a discreet button on the outside of your abdomen. After each meal, the device enables you to empty, or "aspirate", up to 30% of your meal into the toilet through this tube by connecting a small, handheld device to the button. The device is about the size of a smartphone, and stores away in a small case afterwards. The process takes 5 to 10 minutes.
Patients can usually return home within one to two hours, and
many return to work very quickly compared to invasive
bariatric surgeries.
FDA approval was based on data from a study in which 111 patients
received treatment with AspireAssist in combination wi th proper
lifestyle therapy, including
nutrition and
exercise counseling, and 60 patients who were treated only with
lifestyle therapy. At 12 months follow-up, patients using the
AspireAssist device lost a average 12.1 percent of body
weight compared to 3.6 percent for the patients who were not
treated with the device.
Both groups of patients had small improvements in obesity-related
conditions, including
hypertension, diabetes, and quality of life. These improvements
may be associated to the lifestyle therapy.

This therapy is used in conjunction with lifestyle counseling. This
program needs to be combined with one-on-one counseling and group
support meetings to encourage healthier food choices, smaller
portion sizes, and increased physical activity. The therapy also
requires careful and comprehensive monitoring by a physician to
ensure you are
losing weight in a healthy manner.
Important Information
According to the FDA release, patients need to be closely
monitored by a health professional during treatment with
the AspireAssist to shorten the tube as they lose weight and
abdominal width, to monitor the use of the device and weight loss,
and to offer lifestyle therapy counseling.
The device should not be used by patients who are pregnant
or lactation, who have
eating disorders (diagnosed bulimia and binge eating disorder,
night eating syndrome), uncontrolled hypertension, inflammatory
bowel disease, stomach ulcers or certain previous abdominal
surgeries, and those with a history of cardiovascular or pulmonary
disease, chronic abdominal pain, coagulation disorders, or at an
elevated risk of clinical complications from an endoscopic
procedure.
Ref:
http://obesitynewstoday.com/
