|
|
|
 , ,
Font size |
Top 10 Worst and Dangerous Incurable Diseases in the world
 Progeria Progeria
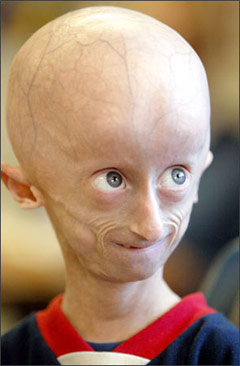 Progeria
is a disease that causes children to age very prematurely and quickly. A baby
with the genetic mutation is born a normal, healthy baby but symptoms normally
appear when the child is 18-24 months. Children with progeria have bodies like
those of elderly people, with wrinkled skin and poor eyesight and very few
sufferers live beyond 13 years. Progeria
is a disease that causes children to age very prematurely and quickly. A baby
with the genetic mutation is born a normal, healthy baby but symptoms normally
appear when the child is 18-24 months. Children with progeria have bodies like
those of elderly people, with wrinkled skin and poor eyesight and very few
sufferers live beyond 13 years.
Progeria (Hutchinson-Gilford Progeria Syndrome, HGPS, Progeria syndrome) is an
extremely rare genetic disease wherein symptoms resembling aspects of aging are
manifested at a very early age. Progeria is one of several progeroid syndromes.
The word progeria comes from the Greek words "pro" (p??), meaning "before" or
"premature", and "geras" (???a?), meaning "old age". The disorder has a very low
incident rate, occurring in an estimated 1 per 8 million live births. Those born
with progeria typically live to their mid teens to early twenties. It is a
genetic condition that occurs as a new mutation, and is rarely inherited.
Although the term progeria applies strictly speaking to all diseases
characterized by premature aging symptoms, and is often used as such, it is
often applied specifically in reference to Hutchinson-Gilford Progeria Syndrome
(HGPS).
Scientists are particularly interested in progeria because it might reveal clues
about the normal process of aging. Progeria was first described in 1886 by
Jonathan Hutchinson. It was also described independently in 1897 by Hastings
Gilford. The condition was later named Hutchinson-Gilford Progeria Syndrome.
Children with progeria usually develop the first symptoms during their first few
months. The earliest symptoms may include a failure to thrive and a localized
scleroderma-like skin condition. As a child ages past infancy, additional
conditions become apparent usually around 18�24 months. Limited growth,
full-body alopecia, and a distinctive appearance (a small face with a shallow
recessed jaw, and a pinched nose) are all characteristics of progeria. Signs and
symptoms of this progressive disease tend to get worse as the child ages. Later,
the condition causes wrinkled skin, atherosclerosis, kidney failure, loss of
eyesight, hair loss, and cardiovascular problems. Scleroderma, a hardening and
tightening of the skin on trunk and extremities of the body, is prevalent.
People diagnosed with this disorder usually have small, fragile bodies, like
those of elderly people. The face is usually wrinkled, with a larger head in
relation to the body, a narrow face and a beak nose. Prominent scalp veins are
noticeable (made more obvious by alopecia), as well as prominent eyes.
Musculoskeletal degeneration causes loss of body fat and muscle, stiff joints,
hip dislocations, and other symptoms generally absent in the non-elderly
population. Individuals do usually retain normal mental and motor development.
In normal conditions, the LMNA gene codes for a structural protein called
prelamin A. There is a farnesyl functional group attached to the
carboxyl-terminus of its structure. The farnesyl group allows prelamin A to
attach temporarily to the nuclear rim. Once the protein is attached, the
farnesyl group is removed. Failure to remove this farnesyl group, permanently
affixes the protein to the nuclear rim. Without its farnesyl group, prelamin A
is referred to as lamin A. Lamin A, along with lamin B and lamin C, make up the
nuclear lamina, which provides structural support to the nucleus.
Before the late 20th century, research on progeria yielded very little
information about the syndrome. In 2003, the cause of progeria was discovered to
be a point mutation in position 1824 of the LMNA gene, in which cytosine is
replaced with thymine. This mutation causes transcription of the LMNA gene to
stop too early, which results in the creation of an abnormally short mRNA
transcript. This mRNA strand, when translated, yields an abnormal variant of the
prelamin A protein whose farnesyl group cannot be removed. Because its farnesyl
group cannot be removed, this abnormal protein, referred to as progerin, is
permanently affixed to the nuclear rim, and therefore does not become part of
the nuclear lamina. Without lamin A, the nuclear lamina is unable to provide the
nuclear envelope with adequate structural support, causing it to take on an
abnormal shape. Since the support that the nuclear lamina normally provides is
necessary for the organizing of chromatin during mitosis, weakening of the
nuclear lamina limits the ability of the cell to divide.
Progerin may also play a role in normal human aging, since its production is
activated in senescent wildtype cells. Unlike "accelerated aging diseases" (such
as Werner's syndrome, Cockayne's syndrome, or xeroderma pigmentosum), progeria
is not caused by defective DNA repair. Because these diseases cause changes in
different aspects of aging, but never in every aspect, they are often called
"segmental progerias".
Diagnosis is suspected according to signs and symptoms, such as skin changes,
abnormal growth, and loss of hair. A genetic test for LMNA mutations can confirm
the diagnosis of progeria.
No treatments have been proven effective. Most treatment focuses on reducing
complications (such as cardiovascular disease) with heart bypass surgery or
low-dose aspirin. Children may also benefit from a high-energy diet. Growth
hormone treatment has been attempted. The use of morpholinos has also been
attempted in order to reduce progerin production. Antisense Morpholino
oligonucleotides specifically directed against the mutated exon 11�exon 12
junction in the mutated pre-mRNAs were used.
A type of anticancer drug, the farnesyltransferase inhibitors (FTIs), has been
proposed, but their use has been mostly limited to animal models. A Phase II
clinical trial using the FTI lonafarnib began in May 2007. In studies on the
cells another anti-cancer drug, rapamycin, caused removal of progerin from the
nuclear membrane through autophagy. It has been proved that pravastatin and
zoledronate are effective drugs when it comes to the blocking of farnesyl group
production. However, it is important to remember that no treatment is able to
cure progeria.
Farnesyltransferase inhibitors (FTIs) are drugs which inhibit the activity of an
enzyme needed in order to make a link between progerin proteins and farnesyl
groups. This link generates the permanent attachment of the progerin to the
nuclear rim. In progeria, cellular damage can be appreciated because that
attachment takes place and the nucleus is not in a normal state. Lonafarnib is
an FTI, which means it can avoid this link, so progerin can not remain attached
to the nucleus rim and it now has a more normal state. The delivery of
Lonafarnib is not approved by the US Food and Drug Administration (FDA).
Therefore, it can only be used in certain clinical trials. Until the treatment
of FTIs is implemented in progeria children we will not know its effects�which
are positive in mice.
 Pravastatin, traded as Pravachol or Selektine, is included in the family of
statins. As well as zoledronate (also known as Zometa and Reclast, which is a
bisphosphonate), its utility in HGPS is the prevention of farnesyl groups
formation, which progerin needs to provoke the disease. Some animal trials have
been realized using FTIs or a combination of pravastatin and zoledronate so as
to observe whether they are capable of reversing abnormal nuclei. The results,
obtained by blinded electron microscopic analysis and immunofluorescence
microscopy, showed that nucleus abnormalities could be reversed in transgenic
mice expressing progerin. The reversion was also observed in vivo�cultured cells
from human subjects with progeria�due to the action of the pharmacs, which block
protein prenylation (transfer of a farnesyl polypeptide to C-terminal cysteine). Pravastatin, traded as Pravachol or Selektine, is included in the family of
statins. As well as zoledronate (also known as Zometa and Reclast, which is a
bisphosphonate), its utility in HGPS is the prevention of farnesyl groups
formation, which progerin needs to provoke the disease. Some animal trials have
been realized using FTIs or a combination of pravastatin and zoledronate so as
to observe whether they are capable of reversing abnormal nuclei. The results,
obtained by blinded electron microscopic analysis and immunofluorescence
microscopy, showed that nucleus abnormalities could be reversed in transgenic
mice expressing progerin. The reversion was also observed in vivo�cultured cells
from human subjects with progeria�due to the action of the pharmacs, which block
protein prenylation (transfer of a farnesyl polypeptide to C-terminal cysteine).
The authors of that trial add, when it comes to the results, that: �They further
suggest that skin biopsy may be useful to determine if protein farnesylation
inhibitors are exerting effects in subjects with HGPS in clinical trials�.
Unlike FTIs, pravastatin and zoledronate were approved by the U.S. FDA (in 2006
and 2001 respectively), although they are not sold as a treatment for progeria.
Pravastatin is used to decrease cholesterol levels and zoledronate to prevent
hypercalcaemia.
Rapamycin, also known as Sirolimus, is a macrolide. There are recent studies
concerning rapamycin which conclude that it can minimize the phenotypic effects
of progeria fibroblasts. Other observed consequences of its use are: abolishment
of nuclear blebbing, degradation of progerin in affected cells and reduction of
insoluble progerin aggregates formation. All these results do not come from any
clinical trial, although it is believed that the treatment might benefit HGPS
kids. A 2012 study showed that the cancer drug Lonafarnib can be used to treat
progeria. It should always be taken in account that no treatment is delivered in
order to cure Hutchinson-Gilford progeria syndrome; all potential drugs are in
pre-clinical stages.
Several discoveries have been made that have led to greater understandings and
perhaps eventual treatment for this disease. A 2003 report in Nature said that
progeria may be a de novo dominant trait. It develops during cell division in a
newly conceived zygote or in the gametes of one of the parents. It is caused by
mutations in the LMNA (lamin A protein) gene on chromosome 1; the mutated form
of lamin A is commonly known as progerin. One of the authors, Leslie Gordon, was
a physician who did not know anything about progeria until her own son, Sam, was
diagnosed at 21 months. Gordon and her husband, pediatrician Scott Berns,
founded the Progeria Research Foundation.
Next..
Dated 19 October 2013
|
|
|
|
|
|
|









